The phenomenon of RNA interference (RNAi) was discovered by Andrew Fire and Craig Mello, who subsequently won the 2006 Nobel Prize in Physiology and Medicine with their lecture titled ‘RNA interference – gene silencing by double-stranded RNA’ (NobelPrize.org, 2006). This discovery unveiled a new layer of complexity to gene regulation and consequently sparked the production of a new therapeutic modality – short interfering RNA (siRNA), which utilised this newly discovered process.
The production of siRNA involves the use of a ‘guide’ RNA strand to help target a protein complex – the RNA-induced silencing complex (RISC) to a target mRNA to facilitate its cleavage and degradation – therefore silencing any activity of its protein products (Svoboda, 2020). This technique allows the down-regulation of gene products through a simpler determination of the genetic sequence, rather than the previous (and more convoluted) method of developing molecules or antibodies that inhibit a complex 3D protein structure.
Indications treated
There is great therapeutic interest and utility in siRNA due to its requirement of binding transcripts with perfect or near-perfect complementarity, resulting in highly specific targeting. As of June 2025, seven siRNA drugs have been approved by the Food and Drug Administration (FDA) and five by the European Medicine Agency (EMA). The first marketed siRNA therapeutic was Patisiran (ONPATTRO™), which targets mutant transthyretin (TTR) in patients with transthyretin-mediated amyloidosis (hATTR) caused by amyloid fibril formation by the misfolded mutant protein. While this drug uses cholesterol-modified lipid nanoparticles to aid delivery to the liver, later siRNA drugs have used N-acetylgalactosamine (GalNAc) conjugation, which allows uptake via endocytosis through binding to the liver-expressed asialoglycoprotein receptor (ASGR1). This approach is used by Lumasiran (OXLUMO™) to treat the orphan disease primary hyperoxaluria type 1 (PH1), which reduces levels of hepatic glyoxylate oxidase (GO) to combat kidney and bladder stone occurrence.
Other siRNA drugs to target various parts of the body are currently under development. Therapies to the eye can be delivered topically or via intravitreal injection, and the latter has been used to administer siRNA drugs for age-related macular degeneration (Kaiser et al., 2010) and diabetic retinopathy (Jiang & Chen, 2017). Inhaled siRNA formulations have also been developed for treating respiratory diseases, including influenza virus infection (Jamali et al., 2018), and for co-delivery with anti-cancer agents in nanoparticles for multidrug-resistant lung cancer (Patel et al., 2021).
Patent considerations
Inventions surrounding siRNA usually fall into one of two categories. Sometimes, a novel target gene – or a more effective region of a known target gene – is identified, and the main inventive concept is the target sequence where siRNA constructs have complementarity. In other cases, existing siRNAs are modified for increased efficacy, bioavailability and safety; therefore, the invention may involve new chemical modifications or delivery systems.
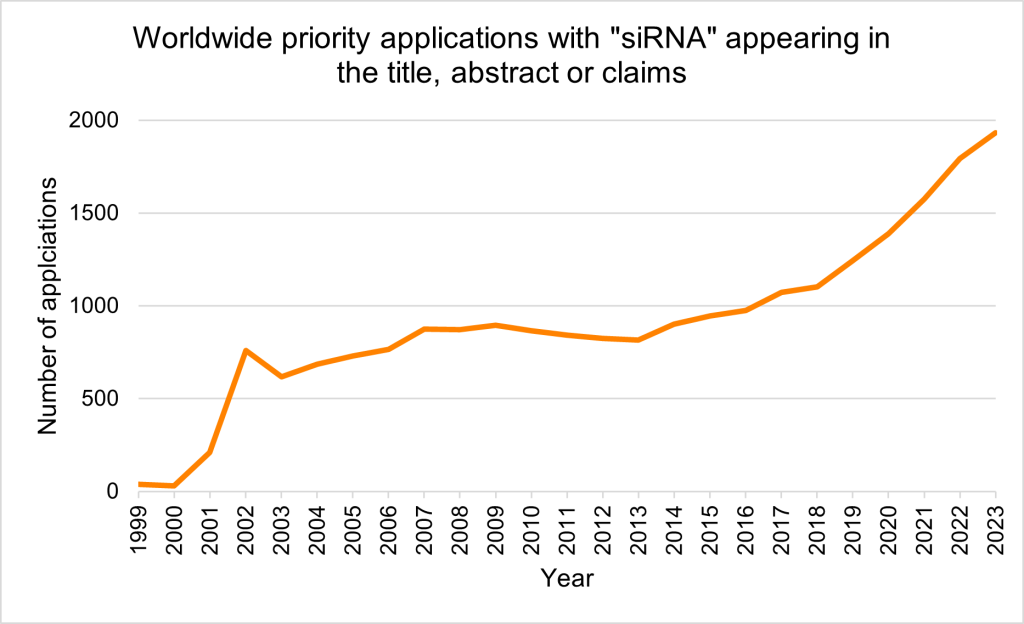
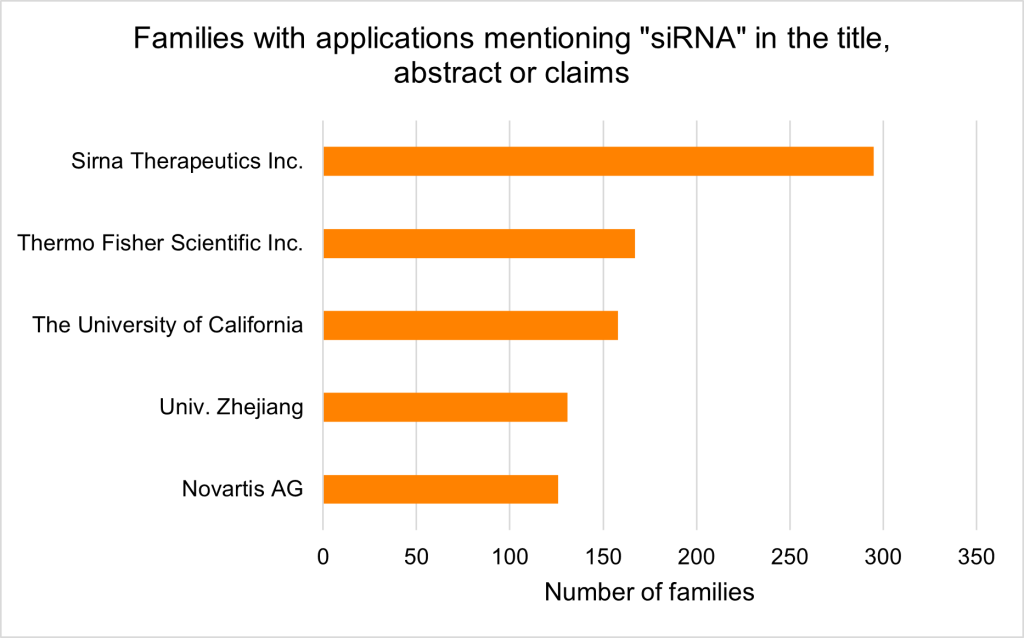
Searching priority patent applications filed worldwide (PatBase) that include the term ‘siRNA’ in the title, abstract or claims uncovers a clear upwards trend in application volume, with initial filings occurring shortly after the discovery of the mechanism in the 1990s (Figure 1). As of August 2025, top assignees include Sirna Therapeutics Inc., Thermo Fisher Scientific Inc. and the University of California (Figure 2).
Relevant EPO guidance
Patent applications for siRNA inventions often contain a multitude of oligonucleotide constructs targeting various regions of a target gene produced by a ‘gene walk’ approach in developing interfering nucleic acids towards that gene. However, when these are claimed in their entirety, it often results in a lack of unity objection before the EPO, as there is no single inventive concept unifying the subject matter. Unification may be evidenced by the presence of a ‘special technical feature’ common to each oligonucleotide. This feature may be absent in such a group of oligonucleotides, for example if they differ in the target gene region or in their chemical modifications. A lack of unity objection may also arise if other sequences targeting the same gene have previously been disclosed or if the chemical modifications of the claimed oligonucleotides are considered standard or routine – and, therefore, do not constitute a distinguishing technical feature.
Such claims will normally be restricted to one of two categories: oligonucleotides that target a specific region of a gene; or oligonucleotides that share a common chemical modification pattern. The unifying feature may be a greater knockdown compared to random target regions or a new chemical modification that produces a consistent pharmacokinetic effect.
The goalposts defining what is deemed inventive shift as technology develops and certain procedures and methods become more commonplace. As is the case with antibodies, where raising antibodies towards a known target antigen of interest is not seen as inventive, developing siRNA oligonucleotides complementary to a known target gene of interest is also a routine task for a person skilled in the art. When arguing that the chemical modifications constitute the inventive concept of a set of oligonucleotides, general modifications to oligonucleotides that are widely known to improve the efficacy, bioavailability and safety of siRNA therapeutics – such as common patterns of modifications involving phosphorothioate backbones, 2’-O-methyl sugars and 3’- and/5’-end caps – are seen as an obvious choice. Thus, claims must be directed towards more specific features that offer an improvement over siRNAs known in the art. Early siRNA inventions had allowed claims directed towards features, such as their complementarity, to a target gene, length and presence of a certain number of backbone or sugar modifications. Now, these features would be seen as obvious, and current siRNA claims are much more complex, often defining modifications at each residue position and claiming the nucleotide sequence of the siRNA itself rather than defining it simply by its target gene.
When it comes to modified siRNAs, in particular, it is important to define the siRNAs beyond the individual modified constructs. General formulas may be used to cover a wide range of nucleic acids with similar modification patterns or to provide a selection of certain modifications. This approach allows broader initial claims that encompass many options of nucleic acid constructs (which can be argued all share a technical advantage) but provides the option to amend to more narrow subject matter if required.
When arguing for the inventiveness of an siRNA invention, comparative data is essential to show a technical advantage of the claimed subject matter over the prior art. Such data is key in linking the claimed invention to a technical effect. An ideal dataset would include experiments with both relevant controls and data generated from prior art siRNAs looking to solve the same or similar technical problem to compare with the siRNAs of the invention.
Summary
While claims towards siRNA therapeutics are becoming increasingly narrower, patenting of siRNA inventions remains possible with sufficient data, particularly that which compares the invention to the relevant prior art. It is key to demonstrate a technical effect linked to at least one unifying feature, which may allow claims encompassing a number of siRNA constructs. Developing and executing a robust IP strategy is essential for entities working on RNAi therapeutics to ensure successful applications and maximum protection of their intellectual property. The biotech team at GJE has extensive experience in patenting nucleotide-based therapeutics. To discuss your biotech IP strategy, please contact us at biotech@gje.com.
References
Advanced information – Nobel Prize in Physiology or Medicine 2006. Available online.
Kaiser, P. K., Symons, R. C., Shah, S. M., Quinlan, E. J., Tabandeh, H., Do, D. V., Reisen, G., Lockridge, J. A., Short, B., Guerciolini, R., Nguyen, Q. D., & Sirna-027 study investigators. (2010). RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am J Ophthalmol, Jul 150(1), 33–39.e2. Available online.
Jamali, A., Mottaghitalab, F., Abdoli, A., Dinarvand, M., Esmailie, A., Kheiri, M. T., & Atyabi, F. (2018). Inhibiting influenza virus replication and inducing protection against lethal influenza virus challenge through chitosan nanoparticles loaded by siRNA. Drug Deliv Transl Res., Feb 8(1),12–20. Available online.
Jiang, S., & Chen, X. (2017). HMGB1 siRNA can reduce damage to retinal cells induced by high glucose in vitro and in vivo. Drug Des Devel Ther., Mar 15(11), 783–795. Available online.
Patel, V., Bardoliwala, D., Lalani, R., Patil, S., Ghosh, S., Javia, A., & Misra, A. (2021). Development of a dry powder for inhalation of nanoparticles co-delivering cisplatin and ABCC3 siRNA in lung cancer. Ther Deliv., Sep 12(9), 651–670. Available online.
Svoboda, P. (2020). Key mechanistic principles and considerations concerning RNA interference. Front. Plant Sci., 11, 1237. Available online.