Technology overview
Microbiome patenting refers to the practice of patenting innovations related to the microbiome, which is the collection of microorganisms, such as bacteria, fungi, viruses, and other microbes, that live in and on humans, animals, plants, and the environment (Hoffmann, 2016). In recent years, microbiome-related patents and applications have become a hot topic as research into the microbiome has exploded, with implications for health and disease treatment, biotechnology, and the environment.
While the term ‘microbiome’ refers to the collective and dynamic genomes of microorganisms, there are various types of microbiomes, including those associated with the skin, mucosae, plants, and environment, as well as synthetic microbiomes. Specifically, the gut microbiome has attracted considerable attention in the patent landscape due to its potential to be modulated to treat and prevent a broad range of diseases and enhance overall health.
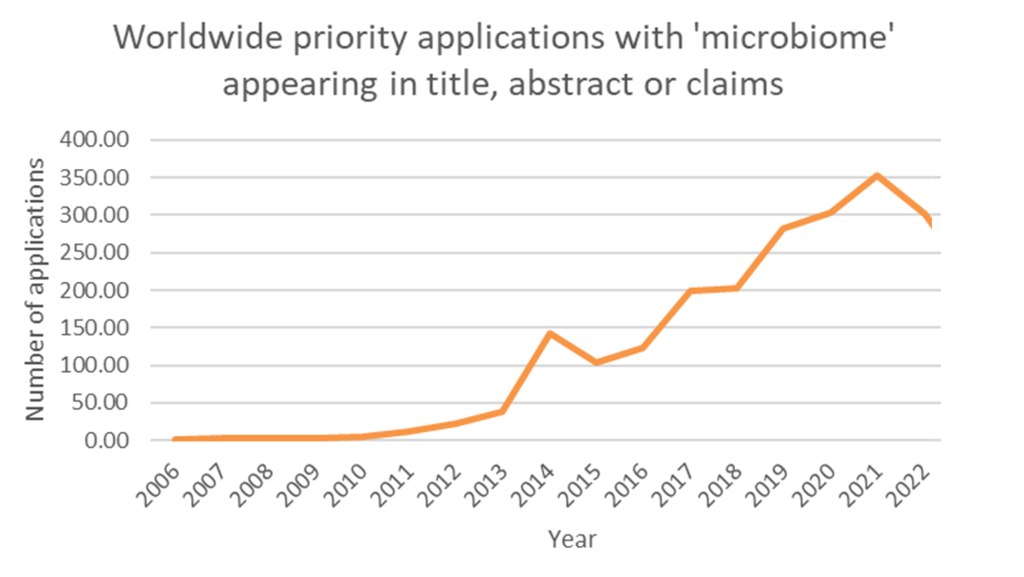
Key players include Ubiome Inc., Psomagen Inc., the University of California and Nestle SA (Patbase, 2025). While there has been a steady increase in priority applications mentioning ‘microbiome’ in the title, abstract, or claims from 2012 onward, a slight decline has been observed for 2022.
Indications treated
Microbiome patents are being sought for innovations having the potential to treat a variety of diseases and medical conditions, particularly those related to the gut microbiome. Conditions that are being treated or managed through microbiome-based therapies include infectious diseases such as Clostridium difficile infection and/or antibiotic-resistant infections. Other conditions include gastrointestinal diseases such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD), metabolic diseases (e.g., type 2 diabetes) and non-alcoholic fatty liver disease (NAFLD), cancers, and autoimmune diseases.
For instance, faecal microbiota transplants or therapy (FMT) is one of the most well-established microbiome therapies, particularly for C. difficile infections that do not respond to antibiotics (Wang et al., 2025). In some cases, patients who do not respond to cancer immunotherapy may benefit from FMT to improve their immune system’s ability to attack cancer cells (Yang et al., 2024). Nevertheless, concerns about the safety, efficacy and precision of FMT still exist (Bibbò et al., 2020).
Patent considerations
Patenting in the field of microbiomes ranges from small molecule drugs and nutritional compositions to precision medicine and big data. Accordingly, patents are being pursued in several areas within the microbiome space. Examples include compositions, microbiome-related therapies (e.g. probiotics), methods for modifying the microbiome, diagnostic techniques, genetic sequencing and bioinformatics tools, microbial engineering, and natural product patents for novel compounds produced by microbes with potential therapeutic applications.
Patenting microbiome-related inventions presents several challenges due to the unique nature of microbiomes and the complexities involved in their study and manipulation.
As for all inventions, to be patentable, microbiome-related inventions must be novel and non-obvious. Notably, microbiomes are highly complex and dynamic, and their composition can vary significantly between individuals, environments, and even over time. For instance, live bacteria are extremely complex and far more complex than small molecules or antibody therapeutics. This makes it difficult to define clearly and describe microbiome-related inventions in a way that satisfies patenting requirements. Patents must therefore be drafted with clear and precise language, detailing specific microbial compositions, specific microbial strains, methods of treatment, or modifications to the microbiome. Providing detailed data, experimental results, and clear claims is essential to demonstrate how the invention works and its potential applications.
Relevant EPO guidance
Patent claims often relate to, for example, specific strains, formulations of microbes, methods of treatment or use, diagnostics or methods of production. That is, claims may relate to a ‘composition comprising a specific bacterial strain deposited under a certain accession number X’ with medical use claims and method claims limited to the specific deposited strains. Notably, patent claims directed to bacterial strains almost always refer to a deposit number. If a biological deposit is being relied on to support a patent application, then it is essential that the deposit is made in a suitably recognised institution before the patent application is filed.
While microbiome-based treatments, such as probiotics or FMT, are being developed, the exact mechanisms of action by which they work are often not fully understood. This lack of clarity can make it challenging to explain and defend a microbiome-related invention’s effectiveness in a patent application. Companies should aim to gather as much experimental evidence as possible to demonstrate the invention’s efficacy and the mechanism by which it produces therapeutic effects to help overcome objections related to lack of support. However, it may not be necessary to understand the mechanism of action fully as long as you can demonstrate that the effects are reproducible and the methods are fully explained. It is possible that you may need to rely on method claims in such instances, rather than product claims but this will depend on the details.
When it comes to patenting, the more data the better, but the costs and time involved in obtaining this should be balanced against other needs of the business. Nevertheless, the data in the application must make the invention credible. In a well characterised field, where processes are well understood, you may get away with filing an application earlier if you have at least some data to support your claims. In the case of more speculative applications, it would be more sensible to wait until more data are available.
One particularly tricky hurdle to get over when claiming a product for use in a medical method of treatment in this field is the difficulties in defining the product. If the product is the result of the culture of a population of organisms under certain conditions, then you may not be able to define the final product clearly. This makes claiming medical uses in the usual format of “product X for use in treating disease Y” challenging. You may have to rely on claiming the use of a product defined by its method of manufacture, which is not always acceptable at the EPO. It may be necessary to rely on the method of manufacture of the product in your patent, rather than the uses of the product. Nevertheless, the details of the particular treatment will be important here and advice should be sought from your patent attorney.
To show that the invention is based on an inventive step, it is recommended to include comparative experimental data (e.g., EP3801566). Specifically, the use of a combination of two or more active ingredients with known identical therapeutic use can only be considered inventive when a surprising effect, a synergistic effect for example, can be assigned in relation to the claimed therapeutic use.
Navigating patent eligibility challenges in the US
Patent laws vary significantly between countries, and certain aspects of microbiome inventions may be patentable in one jurisdiction but not another. One concern in the microbiome space is the increasingly stringent practice at the US Patent Office (USPTO) regarding applications claiming naturally occurring products, such as bacteria, as well as methods based on natural phenomena or diagnostic methods, which risk being deemed patent-ineligible. However, this challenge should not pose a significant barrier to building robust patent portfolios and establishing a business presence in the US as there will usually be a suitable strategy to pursue to patent any given innovation.
To date, the field of microbiome inventions is relatively unchartered territory with little case law, regulatory uncertainty and a need for detailed formulations. Specifically, in the US, it may be difficult to claim natural products, as many microbiome based inventions are likely to be viewed by the USPTO as falling under patentability exceptions such as laws of nature (metabolite production and effect on host), natural phenomena (microbial distribution in gut and on skin) and/or abstract ideas (genomic data analytics). However, if you have developed a new microbiome-related medicament where you have demonstrated an unexpected advantage or efficacy and you have shown that this is repeatable, then there is likely to be a suitable strategy available for protecting this with a patent application. The details of the application will probably differ depending on the country in which it is examined so the application must be drafted with this in mind but there will probably be a strategy that can be pursued.
Summary
The microbiome patent landscape is still evolving. In recent years, there has been a significant increase in filings related to microbiome-based treatments, diagnostics, and biotechnologies. However, defining microbiome inventions remains a challenge in practice. Patent applications in this field must be drafted with global issues in mind and be well supported with experimental data.
The biotechnology group at GJE has extensive experience in patenting a diverse range of biotechnological inventions in addition to advising innovative biotech companies and investors. To discuss your biotech IP strategy, please contact us at biotech@gje.com.
References
Bibbò, S., Settanni, C. R., Porcari, S., Bocchino, E., Ianiro, G., Cammarota, G., and Gasbarrini, A. (2020). Fecal microbiota transplantation: screening and selection to choose the optimal donor. Journal of Clinical Medicine. 9(6), 1757. Available online.
Eli Lilly v. Actavis [2017] UKSC 48. Available online.
Hoffmann, A. R. (2016). The Microbiome: The Trillions of Microorganisms That Maintain Health and Cause Disease in Humans and Companion Animals. SAGE Journals, 10-21. Available online.
Patbase. (May 2025). Retrieved from Patbase.
Wang, X., Zhao, D., Bi, D., Li, L., Tian, H., Yin, F., et al. (2025). Fecal microbiota transplantation: transitioning from chaos and controversial realm to scientific precision era. Science Bulletin, 1-16. Available online.
Yang, Y., An, Y., Dong, Y., Chu, Q., Wei, J., Wang, B., and Hailong, C. (2024). Fecal microbiota transplantation: no longer cinderella in tumour immunotherapy. eBio Medicine, 104967. Available online.