The deadline for medical devices in Europe to fully comply with the latest regulations is fast approaching.
From 26 May 2020, a wide range of devices in the medical industry, including surgical instruments and hospital beds, will have a new set of rules in the Medical Device Regulation (MDR), with rules for in-vitro diagnostic devices to follow in 2022 in the In-Vitro Diagnostic Medical Device Regulation (IVDR).1
Conventionally, regulations are sometimes regarded as constraints that can prevent innovation. The introduction of the MDR and IVDR present an opportunity to see whether the contrary is true and regulation can act as a driving force for innovation.
In particular, there has been interest in whether the new Post-Market Surveillance requirements of the Medical Device Regulation would stimulate technological innovations to meet these requirements.
To give a brief summary, Post-Market Surveillance means that device manufacturers will be required to obtain feedback about devices as they are used in the field in order to identify any problems and quickly take any necessary corrective or preventive actions.
This presents an opportunity for innovators in the growing IoT (Internet-of-Things) sector to work with medical device manufacturers to help improve quality of patient care at the same time as complying with the legislation.
More specifically, IoT is about taking advantage of adding network connectivity to objects which would not traditionally be communication-capable, such as lighting systems, heating systems etc. Medical devices are not traditionally communication-capable, but Post-Market Surveillance requires exactly the sort of data gathering that IoT can provide.
For example, a surgical tool may be provided with means to log key parameters for evaluating how successfully it is achieving its intended purposes. These data, appropriately anonymised in view of confidentiality requirements, can then be reported to and analysed by the manufacturer.
But have the regulations had an effect on innovation, or do the regulations simply complement changes that were already taking place in the industry? To try to answer this, we looked into the patent filing statistics for medical IoT technology.
Global Medical IoT filings (1999-2017)
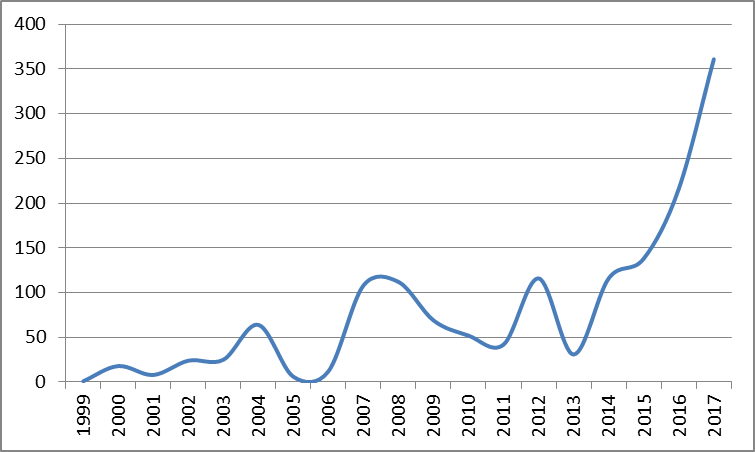
As shown in the graph above2, the number of medical IoT applications more than doubled in 2017 year-on-year, and approximately tripled by comparison with 2015.
All IoT global filings (1999-2017)
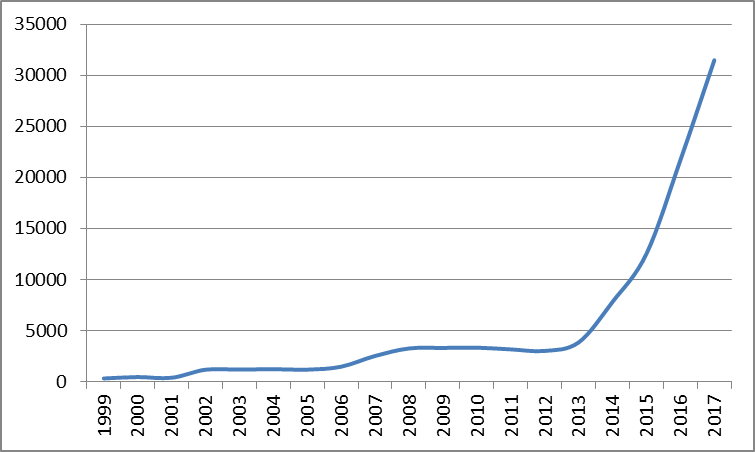
As shown above3, IoT is rapidly rising in many sectors apart from medical devices, and medical IoT is only a small portion of all IoT development. However, growth in medical IoT appears to have outpaced the underlying IoT trend during this period, achieving an average of 16% faster growth than IoT generally over the last 5 years.
This makes a compelling case that Europe’s new medical device regulations are stimulating innovation and that, more generally, patentable innovations may also be expected in response to the introduction of future technological regulations.
Of course, we can only look at the innovation that has already happened. The MDR and IVDR are still new, and it may be that the greatest innovations stimulated by this regulatory framework are yet to be seen.
In order to fully benefit from your innovations, and prevent competitors from using your ideas, you should consider obtaining patent protection. If you would like advice on protecting your medical device or IoT innovation, contact James at James.PrankerdSmith@gje.com.
1For more information about the medical device regulations, go to https://www.gov.uk/guidance/medical-devices-eu-regulations-for-mdr-and-ivdr or consult the regulatory agency in your country.
2Graph represents global applications with A61 classification and which mention IoT. Obtained using PatBase.
3Graph represents all applications which mention IoT. Obtained using PatBase.


