Technology overview
Bispecific monoclonal antibodies (BsAbs) are antibody constructs that create additional strategies in designing therapeutic avenues due to their ability to bind two different targets as a single molecule. BsAbs may bind two different antigens or two different epitopes on the same antigen. When binding to a single antigen, BsAbs provide advantages over their monospecific counterparts, as two unique epitope-binding sites provide increased avidity and reduced antigen escape due to two sites being recognised. BsAbs directed towards two different antigens are often designed to provide synergistic effects, such as the crosslinking of two receptors to induce a signalling cascade, or to activate T cells assisting the formation of an immunological synapse between a T cell and an antigen (also known as T cell engagers).
BsAbs come in two different forms: Immunoglobulin G (IgG)-like and fragment-based. Developmental platforms to produce IgG-like BsAbs generally involve modifications of the heavy chain’s chemistry and spatial structure (specifically, the fragment crystallisable Fc region) to promote heterodimerisation of the two different heavy-light chain halves of the antibody together, which is a key challenge in creating BsAbs. This challenge is not present in fragment-based BsAbs, since they lack the Fc region of the heavy chain. Instead, fragment-based BsAbs comprise either fragment antigen-binding (Fab) regions or single chain variable fragments (scFv) that have been chemically linked. Specific developmental platforms for BsAbs are reviewed here.
Indications treated
Understandably, BsAbs are of great interest in oncology and immunology, being a new developmental product stemming from monospecific antibodies that have previously been and are currently being used and engineered to treat a wide range of indications. In 2015, Blinatumomab (Blincyto™) became the first BsAb to be approved by the European Medicines Agency (EMA) to treat precursor B-cell acute lymphoblastic leukaemia (ALL). Mechanistically, Blincyto™ is a bispecific T-cell engager (BiTE) and binds to both B-cell lineage expressed CD19 and T-cell expressed CD3 to form a cytolytic synapse and promote tumour cell lysis and apoptosis. BsAbs have also been developed towards autoimmune, neurodegenerative and blood-related disorders. Emicizumab (Hemlibra™) is used to treat haemophilia A, a condition resulting from a clotting factor VIII deficiency that causes bleeding susceptibility due to the inability of the blood to clot. Hemlibra™ brings together clotting factors IXa and X, mimicking the role of clotting factor VIII.
Patent considerations
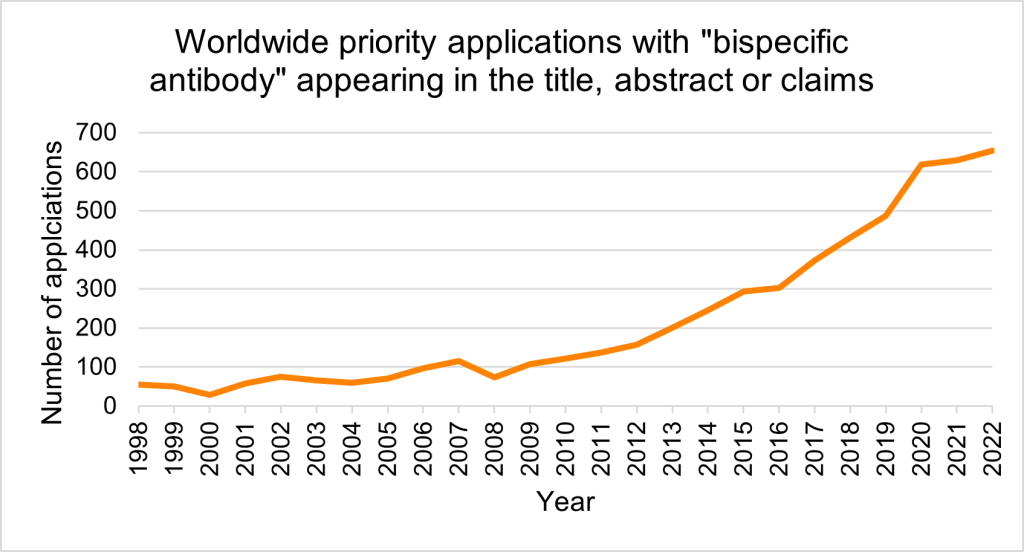
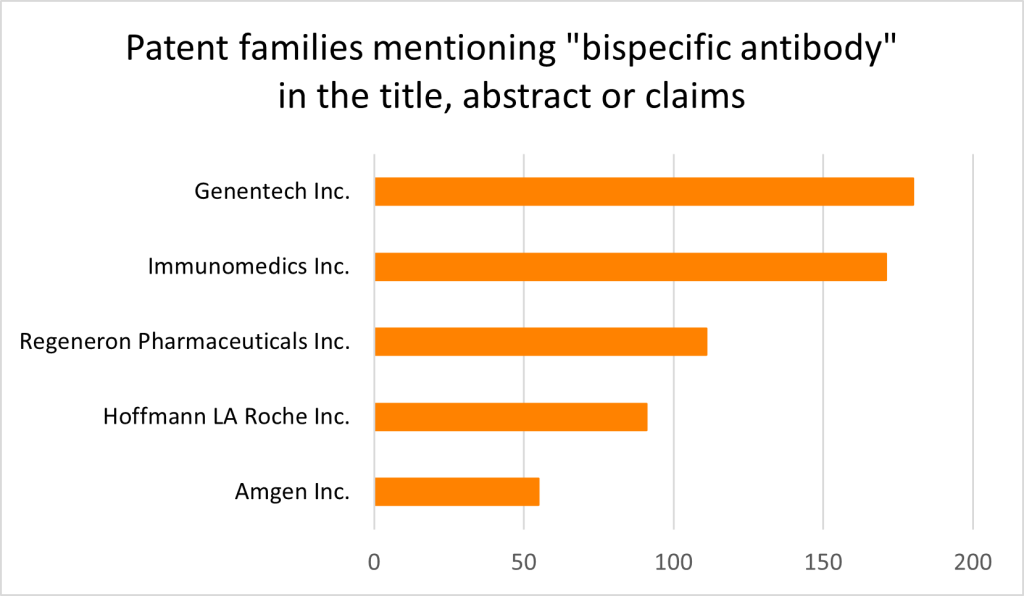
Looking at the trends of priority applications mentioning ‘bispecific antibody’ in the title, abstract or claims, we can see a clear and continued rise in such specifications owing to the therapeutic and commercial interest of BsAbs in recent years (Figure 1, PatBase). The top assignees by number of patent families include Genentech, Immunomedics (now Gilead Sciences) and Regeneron Pharmaceuticals (Figure 2, PatBase).
Antibody inventions must meet the same requirements for patentability as other inventions; however the European Patent Office (EPO) provides specific guidance on the format in which these inventions may be claimed and how these claims should be assessed in the light of the prior art (EPO GL G-II, 6.). The case law around antibody inventions has provided further guidance and is evolving all the time. It is clear that the EPO does not apply the same standard for sufficiency and inventive step to antibody claims vs claims to other biological molecules. This, in part, is down to the EPO’s view that methods for generating and selecting antibodies are routine, that it is the specific variable sequence of the identified antibody that is responsible for the effect, and that this is not easily generalisable to variant sequences. The breadth and form of claims that may be granted for antibodies is therefore now very dependent on the extent of the supporting data in the application demonstrating the advantages of those specific antibodies. If claims are to be generalised to cover antibodies with different sequences binding to the same target, then the data needs to justify the novel technical effect attributable to targeting that particular epitope and the claim must of course define a novel epitope.
Thus, while broader claims towards functional features of antibodies are possible, the specification must provide sufficient data showing that the invention lies in the antibodies binding to a particular target, rather than the invention being the structural features of the antibody (i.e. their amino acid sequence). Claiming an antibody by its function is possible when no prior art antibodies are known to bind the target; however, the data must still show that the antibody has a surprising technical effect in order to meet the inventive step requirement. Claiming an antibody by functional features also requires that the patent specification sufficiently teaches a skilled person how to reproducibly produce antibodies with those features, which requires more data and disclosure than it would for antibodies claimed structurally by, for example, specifying their complementarity determining region (CDR) sequences. In any event, the EPO typically (but not always) requires antibody claims to be limited to CDR sequences (and sometimes also framework sequences) even when no known antibodies bind to the same target. This typically depends both on how the patent application was drafted and on what data and disclosure was provided.
Compared to monospecific antibodies, BsAbs provide additional opportunities for inventive contribution since antibodies with specificity towards two epitopes are not produced in nature. Therefore, there are opportunities to claim additional scope based not just on the particular bi-specificity but also on antibody structure and methods of manufacture. Thus, while simply combining two known binding specificities in a single antibody may not be enough for an inventive claim (although this will again depend on the data), inventive step could be recognised for a novel type of functional antibody format without the need to specify the targets or antibody sequence. These kinds of platform claim can be very valuable. The last two decades have seen continued innovation in BsAb formats, from the IgG-like knobs-into-holes and strand-exchange engineered domain platforms to the more recent fragment approaches such as BiTE and dual affinity retargeting antibodies. Therefore, this particular assessment of antibody inventiveness is of great relevance to BsAbs.
The EPO Boards of Appeal have provided guidance on key aspects of examination towards bispecific antibody inventions which would be useful to consider for applicants looking to file in this field.
T0957/18 gave insight into the inventive step assessment of BsAbs in the light of disclosed monospecific antibodies and highlighted the importance of identifying the technical advantages of providing a bispecific antibody rather than a monospecific combination therapy. In this case, it was decided that an inventive step was present for a particular bispecific antibody, where the prior art had disclosed a combination therapy of two monospecific antibodies towards the same two targets having a synergistic effect. The Board determined that there was no motivation to provide a single medicament as opposed to two separate ones in combination. The technical effect exhibited by the BsAb was a reduction in toxicity through binding to less CD47 (therefore decreasing off-target cell lysis) compared to an anti-CD47 monospecific antibody, and there was no teaching of such in the prior art. Therefore, the bispecific antibody was held to be inventive.
T2159/12 focused on the inventiveness of domain rearrangement in the light of prior art that disclosed similar constructs. Both the invention in question and the prior art concerned antibody constructs comprising CD3 and CD19 heavy and light chains. The application claimed a particular order of CD3 and CD19 heavy and light chains to produce a bispecific construct, this difference in domain rearrangement resulting in higher cytotoxic activity on CD19-expressing NALM 6 cells. The closest prior art disclosed the same domains in a different rearrangement. The closest prior art disclosed bispecific antibody constructs comprising CD3 and CD19 heavy and light chains in a different order to the claimed invention and no indication that altering the domain arrangement would have any an influence on the construct’s cytotoxic activity. Therefore, the prior art contained no teaching towards the rearrangement of domains achieving the technical effect of increased cytotoxic activity, and the presence of an inventive step was acknowledged.
Summary
BsAbs are a fascinating technology that has furthered therapeutic approaches in the oncologic and immunologic fields. While the key considerations of antibody patentability apply, the increased structural complexity of bispecific antibodies produces further opportunities for patenting new antibody formats, binding combinations and methods of production. As BsAbs continue to advance in their clinical use, securing strong intellectual property protection remains critical to secure investment and ensure the successful commercialisation of this biotherapeutic modality. An up-to-date and detailed knowledge of the requirements is crucial when looking to patent BsAbs in Europe.
The biotechnology group at GJE has extensive experience in patenting a diverse range of biotechnological inventions in addition to advising innovative biotech companies and investors. To discuss your biotech IP strategy, please contact us at biotech@gje.com.
References
Ma, J. et al. 2021. Bispecific Antibodies: From Research to Clinical Application. Frontiers in Immunology. 12:626616. doi:10.3389/fimmu.2021.626616
Blincyto | European Medicines Agency (EMA)
Hemlibra | European Medicines Agency (EMA)
T 0957/18 (Synergistic anti-CD47 therapy/LELAND STANFORD JUNIOR UNIVERSITY) 24-09-2020 | epo.org


