Technology review
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) has revolutionised the gene and cell therapy space. Following the discovery of the CRISPR-Cas9 system in 2012 (Jinek et al., 2012), the idea of editing the human genome to treat previously incurable diseases is no longer science fiction, but a thing of reality. With gene editing previously being a time consuming and laborious task, CRISPR has turned it into a simple “cut and paste” job. Using just two components, a single guide RNA (sgRNA), comprising a target-specific crRNA fused to a tracrRNA, and a CRISPR-associated (Cas) endonuclease, a pair of “molecular scissors” is created, capable of targeting any sequence in the genome.
Applications of CRISPR-based therapeutics
The ability of CRISPR-based therapies to easily edit specific sites within a given genome has bolstered the new frontier of curative medicine. We are now living in an age where diseases previously thought to be untreatable are now, in theory, curable, potentially with a single dose of treatment. In November 2023, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) became the first in the world to approve a CRISPR-based gene therapy, branded as CASGEVY™, for treating sickle cell disease and transfusion-dependent beta thalassemia. However, the very nature of how CRISPR-based therapies work mean the uses of such therapies are truly agnostic in nature. This is evidenced by the range of therapy areas currently in clinical trials with CRISPR-based therapies. These include blood-disorders, protein folding disorders, cancers, bacterial and viral infections, cardiovascular disease, diabetes and autoimmune disorders (Henderson, 2024).
Patent considerations
Many aspects of CRISPR-based technologies are potentially patentable, including the CRISPR-Cas system itself, compositions containing the system, and their medical applications. Like many technologies in the gene therapy space, CRISPR-based therapies are complex, multicomponent products. As such, it is crucial for companies working in this space to conduct regular freedom-to-operate (FTO) exercises to understand properly, and be able to navigate successfully, the intellectual property (IP) landscape surrounding their product. By identifying potential IP risks early on, companies can prevent issues further down the line, including costly legal disputes.
Like all other technologies, any patent applications relating to CRISPR-based therapies should disclose both the broadest and narrowest embodiments possible, with various positions falling in between. It is particularly important to include embodiments of the invention that mirror the intended commercial product. Not only does this ensure that the commercial product will be protected by your patent, but also that there is appropriate basis in your patent application to base any potential supplementary patent certificates (SPCs) on – a mechanism that allows for the extension of protection for a specific patented pharmaceutical product. Such a consideration is particularly relevant in the context of CRISPR-based therapies, where companies in this space bear significant costs to bring said therapies to market.
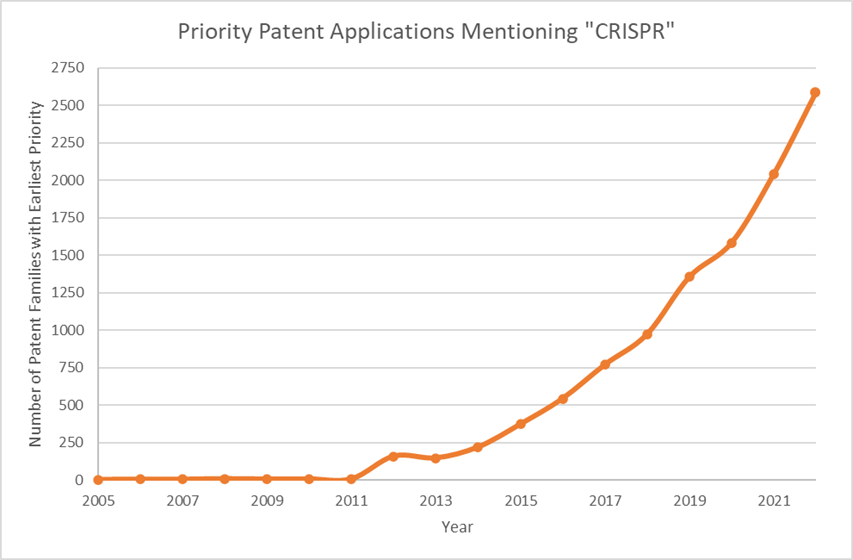
Priority patent applications filed worldwide mentioning “CRISPR” in the title, abstract, or claims have seen a steady increase since the discovery of the CRISPR-Cas9 technology in 2012 (Patbase, accessed 2024). With the approval of CASGEVY™ and further improvements in CRISPR-based technologies, it is expected that such a trend in filing patent applications in this space will continue to rise. It comes as no surprise that amongst the top applicants for CRISPR-based applications are the Broad Institute, The University of California, and the associated companies Jennifer Doudna and Feng Zhang (key players in the field), CRISPR Therapeutics and Editas Medicine, respectively.
Timing of filing
Deciding exactly when to file your patent application is a key challenge in obtaining a granted patent. In the CRISPR-based field, this decision will be determined by the availability of experimental data that supports your claimed invention. In the first instance, this may be in vitro or in silico data that provides proof of concept data, for example, the targeting of a specific gene. This data can then be followed up with more extensive data, for example, in vivo data, preferably within a year of the initial application filing.
Sufficiency and plausibility
At the time of filing, the claimed invention must be considered to be “sufficiently disclosed”, such that a skilled person would be able to reproduce the claimed invention. Therefore, all methods relating to any aspect of the invention should be known at the time of filing and provided in detail in the application as filed. Additionally, experimental data is needed to show that the invention is likely to work, i.e. that it is plausible. If these requirements are not met at the time of filing, you leave your patent application/granted patent open to attack further down the line.
For example, in T 2488/12, a decision based on a patent titled “Use of CRISPR associated genes”, it was stated that the patent did not contain any teaching as to which modifications would be suitable for increasing resistance according to claim 1. As a result, the main request and a number of auxiliary requests were deemed unallowable, leading (at least in part) to the revocation of the patent.
Inventive step
A further requirement for obtaining a patent is that the applicant demonstrates that the claimed invention is not obvious (inventive) over the known prior art. In the CRISPR-based field, experimental data is crucial for demonstrating this requirement. For example, the applicant may include experimental data in the application that shows the claimed invention demonstrates enhanced efficacy, activity or stability. Comparative data with the prior art is therefore highly advisable. Furthermore, inclusion of experimental data in which multiple variants are also shown to be effective can be used to argue for broader claims. Without such data, it is possible that the patent office may force you to narrow your claims to mirror the scope of the experimental data included in the application. However, it is important to appreciate the value of even a narrow claim in this field. As long as the features of the claim cover the intended commercial product, valuable protection can be provided. The breadth that will be allowable will very much depend on what the specific contribution to the field is with your invention. If your contribution is a new modified Cas protein for example, then much broader protection beyond the construct will be possible. If you are using known techniques to target a known genetic condition, then you may have to limit your patent to the specific constructs you have tested.
Exceptions to patentability
Restrictions on patents in Europe to methods performed on the human body mean that patent applications must instead claim the materials used in the methods. Examples would include the gene constructs themselves, the modified cells, the reagents or equipment. Therefore, even though CRISPR therapies often involve the process of extracting and modifying the patient’s own cells (and such a process cannot be patented in Europe), there is always an approach that can be taken to provide effective and commercially relevant protection that circumvents the exclusion of method-of-treatment claims at the EPO.
Summary
CRISPR-based therapies are an exciting new area of clinical medicine, which we have only just begun to see the benefits of. Given the relatively recent approval of CASGEVYTM and the continued enthusiasm for research in this field, the development of CRISPR-based therapies is expected to experience continued growth and innovation. Whilst certain challenges need to be navigated when patenting these types of therapies, valuable protection can be achieved by way of a well-drafted patent application, supplemented by experimental data, and a flexible, bespoke IP strategy.
The biotechnology group at GJE has extensive experience in patenting a diverse range of biotechnological inventions in addition to advising innovative biotech companies and investors. To discuss your biotech IP strategy, please contact us at biotech@gje.com.
References
Martin Jinek et al. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science. 337,816-1 (2012). Available Online.
MHRA authorises world-first gene therapy that aims to cure sickle-cell disease and transfusion-dependent β-thalassemia. Press Release. 16 November 2023. Available online.
Henderson, H. (2024). CRISPR Clinical Trials: A 2024 Update – Innovative Genomics Institute (IGI). Available online.
T 2488/12 – Available online.