Technology review
The impact of gene therapies has been revolutionary to the treatment of genetic disorders and diseases. Unlike traditional pharmaceuticals, which typically treat symptoms, gene therapies address the cause at the genetic level. This modality involves delivering genetic material into a patient’s cells, often to compensate for abnormal or dysfunctional genes (Sung and Kim, 2019). The development and implementation of gene therapies require careful consideration of, for example, vector design, delivery techniques, and target cells to ensure both efficacy and safety.
Gene therapies typically use viral vectors, such as adeno-associated viruses (AAVs) or lentiviruses, to deliver therapeutic genes into target cells (Bulch et al., 2021). Depending on the vector type, the genetic material may integrate into the host genome or remain episomal once inside the target cells. This approach allows for the efficient replacement of faulty or missing genes, the inactivation of malfunctioning ones, and the introduction of new or modified genes.
Indications treated
Gene therapies have demonstrated significant potential in treating various genetic disorders, particularly severe single-gene conditions such as haemophilia, thalassaemia, and cystic fibrosis, which have been primary targets for therapeutic development (Beitelshees et al., 2017). One of the first gene therapies to receive FDA approval was Luxturna® (voretigene neparvovec) in 2017, developed for the treatment of Leber’s congenital amaurosis, a rare genetic disorder that leads to progressive blindness. Luxturna® works by delivering a functional copy of the rpe65 gene to retinal cells to restore vision. In 2022, Hemgenix® (etranacogene dezaparvovec) received FDA approval for the treatment of haemophilia B. This gene therapy uses an Adeno-associated virus (AAV) vector to deliver a functional copy of the f9 gene, enabling patients to produce the missing clotting Factor IX, thereby reducing bleeding episodes and the need for regular infusions of clotting factor concentrates.
Patent considerations
Patent protection is crucial in the development and commercialisation of gene therapies. Patent claims for gene therapies can cover various aspects, including the specific genetic sequences, vectors, delivery methods, compositions, and therapeutic uses.
At the same time, gene therapies often involve complex technologies that span multiple fields, such as molecular biology, virology, and clinical medicine. Hence, Freedom-To-Operate (FTO) analysis is essential to ensure that the development and commercialisation of gene therapies do not infringe on existing third-party rights. Moreover, since many gene therapies use patented vectors and delivery systems, licensing agreements may be necessary.
Over the past two decades, priority patent applications filed globally with references to ‘gene therapy’ in the title, abstract, or claims under International Patent Classification A61 have followed a loosely parabolic growth trend (Figure 1). This pattern mirrors both the setbacks and advancements within the gene therapy field. Notably, the 1999 death of a patient during an adenoviral vector gene therapy clinical trial underscored the critical importance of safety, leading to a decline in clinical trial activity between 1999 and 2015, a trend that was similarly reflected in the number of patent filings during that period (Sibbald, 2001; Goswami et al., 2019).
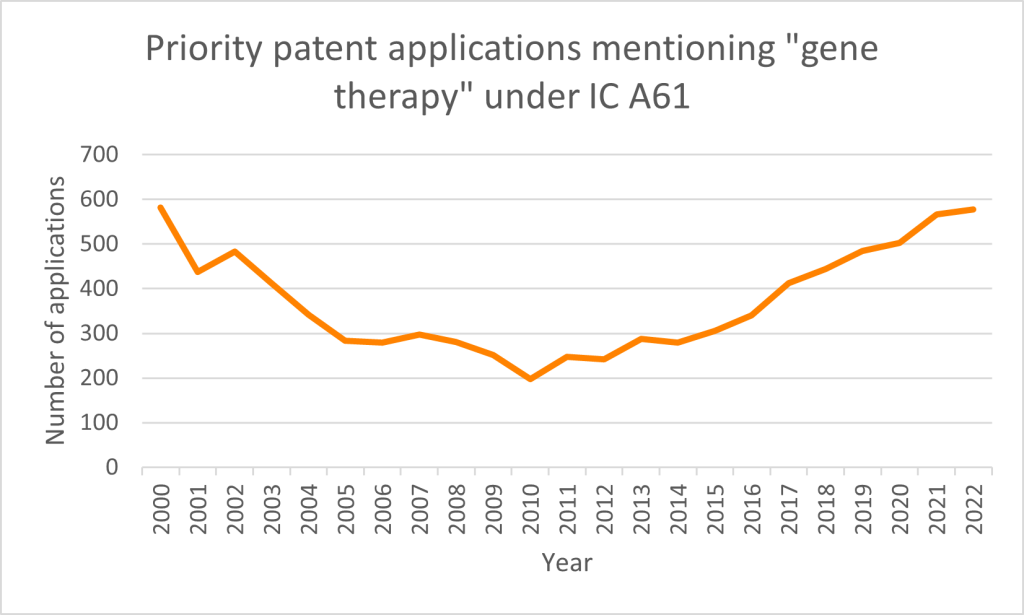
Patent priority filings began their recovery around 2012 and have been linked to two major events. The first was the discovery and development of the CRISPR (clustered regularly interspaced short palindromic repeats)-Cas9 gene-editing system (Jinek et al., 2012). The second was the 2003 approval of Gendicine in China for the treatment of head and neck squamous cell carcinoma (Pearson et al., 2004), and Glybera® (alipogene tiparvovec) by the European Medicines Agency for the treatment of lipoprotein lipase deficiency (Gallaher, 2012).
When patenting gene therapy inventions in Europe, the European Patent Office (EPO) provides multiple avenues for defining genetic sequences and vectors. Claims may be based on structural characteristics, such as specific nucleotide sequences, as well as methods of production or medical uses (EPO GL G-II, 5.2). As some of the vector technologies are more mature, new patent applications tend to focus on the chemistry of the oligonucleotides used in the therapy. Often, the target sequence is well characterised by the time new gene therapies are developed so careful consideration has to be given to the specifics of the intended therapy to craft novel and inventive claims. There remain myriad opportunities for development of strong IP portfolios as many problems are yet to be solved. Chief among these are the challenges in delivering these therapies to target organs and cells in the body. Many companies are working on novel conjugate technologies intended to deliver therapies to specific locations.
Additionally, the concept of plausibility must be carefully considered when filing gene therapy patent applications. This requires that the technical effect (i.e. the specific improvement relating to the new therapy) must have been plausible to a skilled person at the time of filing, based on the original disclosure in the original application. For instance, T 2218/16 concerned an AAV vector comprising a therapeutic gene for use in a method for treating a motor neuron disorder. The opponent argued, inter alia, that the suitability of the AAV vectors for the claimed therapeutic applications was not plausible due to lack of specific data in the patent. The Board confirmed that the provision of evidence in the patent application for a claimed effect was not a prerequisite for patentability, if, based on the data in the patent (application), or on common general knowledge, it was ‘plausible that a product was suitable for the claimed therapeutic applications’ (point 32). The patent was subsequently found to disclose sufficient information on the general availability, production, and mechanism of action of the AAV vector. Neverthelss, this highlights the advantage of having data in your earliest patent application to support the invention.
Summary
Gene therapies represent a transformative approach to treating genetic disorders, offering the potential for long-term and curative outcomes. Patenting gene therapies is complex, necessitating thorough FTO analysis and strategic claim drafting to secure robust patent protection. As the field advances, gene therapies are poised to revolutionise the treatment landscape for a wide range of genetic diseases, providing hope to patients and driving innovation in medical science.
The biotechnology group at GJE has extensive experience in patenting a diverse range of biotechnological inventions in addition to advising innovative biotech companies and investors. To discuss your biotech IP strategy, please contact us at biotech@gje.com.
References
Beitelshees M, Hill A, Rostami P, Jones CH, Pfeifer BA. 2017. Pressing diseases that represent promising targets for gene therapy. Discov Med. 24(134): 313-322. Available online.
Bulcha, J.T., Wang, Y., Ma, H. et al. 2021. Viral vector platforms within the gene therapy landscape. Sig. Transduct. Target. Ther. 6: 53. Available online.
EPO Guidelines, G II-5.2, available online.
Goswami, R. et al. 2019. Gene Therapy Leaves a Vicious Cycle. Front. Oncol. 9: 297. Available online.
Hemgenix® – Available online.
Gallaher J., Gene therapy: Glybera approved by European Commission – BBC News; Available online, accessed April 2025.
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., and Charpentier E. 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 337(6096): 816-21. Available online.
Luxturna® – Available online.
Pearson, S., H. Jia, and K. Kandachi. 2004. China approves first gene therapy. Nat. Biotech.. 22:3-4. Available online.
Sibbald B. 2001. Death but one unintended consequence of gene-therapy trial. CMAJ. 164(11): 1612. Available online.
Sung, Y., and Kim, S. 2019. Recent advances in the development of gene delivery systems. Biomater. Res. 23: 8. Available online.
T 2218/16 – Available online.


