Technology review
Messenger RNA (mRNA) therapies represent a cutting-edge biotherapeutic modality that has gained significant attention for its potential in preventing and treating a wide range of diseases, cancers, and genetic disorders. This technology uses synthetic mRNA molecules engineered to encode specific proteins and leverages the body’s cellular machinery to produce specific peptides or proteins. Once delivered into target cells, these mRNA molecules are translated by ribosomes into functional proteins. This approach mimics the body’s natural protein synthesis process, enabling the production of virtually any desired protein to, for example, enhance protein expression, replace missing proteins, elicit immune responses, or modify cellular processes (Qin et al., 2022).
Key components in the development of mRNA therapies include mRNA sequence design, delivery systems, and modification strategies to enhance stability and translation efficiency. While efficient delivery of mRNA into target cells is a major challenge in the development of mRNA therapies, lipid nanoparticles are the most widely used delivery system, as they protect mRNA from degradation and facilitate cellular uptake (Hou et al., 2021). Other delivery systems include polymers, peptides, and viral vectors, each with unique advantages and challenges. The choice of delivery system depends on the target tissue, required dosage, and specific application of the mRNA therapy.
Indications treated
mRNA therapies have shown promise in a variety of therapeutic areas. The most well-known application is in vaccine development. mRNA subunit vaccines reduce the risk of causing disease in vaccinated individuals and have the advantage of being able to be modified quickly to respond to new variants or pathogens. One example is the widely used mRNA-based COVID-19 vaccines, which were developed and approved at unprecedented speed. These vaccines encode the spike protein of the SARS-CoV-2 virus, eliciting a robust immune response and providing protection against infection (Asrorov et al., 2024).
In cancer therapy, mRNA vaccines are being developed to target tumour-associated antigens. Once administered, the customised mRNA is translated into proteins that mimic these neoantigens, stimulating the immune system, particularly T cells, to recognise and destroy cancer cells expressing the same antigens. One such vaccine is mRNA-4157/V940, an investigational personalised cancer vaccine for the treatment of melanoma (Weber et al., 2024).
mRNA therapy for genetic disorders and rare diseases is another emerging treatment strategy, aiming to restore or replace the function of a missing or defective protein. Current mRNA therapies in clinical development include, for example, mRNA-3705 for the treatment of methylmalonic acidemia (MMA), mRNA-3927 for the treatment of propionic acidemia (PA) and mRNA-3745 for the treatment of glycogen storage disease type 1a (GSD1a) (Baek et al., 2024; Moderna Clinical Trials, 2025).
Patent considerations
Securing robust patent protection ensures exclusive rights for the proprietors and encourages investment in R&D and the advancement and commercialisation of mRNA therapies. Patenting such therapies involves protecting rights related to the use of mRNA technologies for therapeutic purposes, such as vaccines, cancer treatments or gene editing. However, when patenting mRNA technologies, several critical considerations must be addressed to ensure strong, enforceable intellectual property protection.
The interdisciplinary nature of mRNA therapies necessitates comprehensive Freedom-To- Operate (FTO) analysis to navigate existing third-party rights and avoid potential infringements. For instance, many components, such as lipid nanoparticles and modified nucleosides, may be covered by existing patents, requiring careful consideration and potential licensing agreements.
Priority patent applications filed worldwide mentioning ‘mRNA’ in the title, abstract or claims have seen a steady increase in the last 15 years (Figure 1). This increase was initially driven by the main developers in this field, including Genzyme, Chiron, Transgene and CureVac. The outbreak of COVID-19 in early 2020 has been cited as a significant cause of the latest surge in development of mRNA technologies.
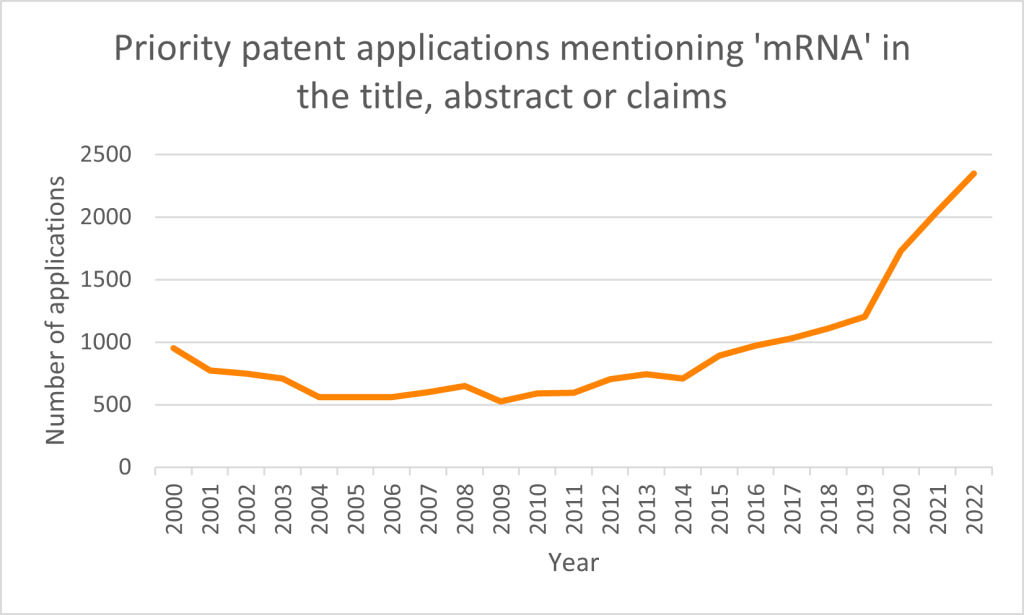
Hence, it does not come as a surprise that Katalin Karikó and Drew Weissman were awarded the Nobel Prize in Physiology or Medicine in 2023 for their discoveries concerning nucleoside base modifications that enabled the development of effective mRNA vaccines against COVID-19.
Relevant European Patent Office guidance
Patent claims for mRNA therapies generally cover aspects relating to nucleic acid sequences and chemical modifications thereof, delivery systems, manufacturing methods, and therapeutic applications. The European Patent Office (EPO) provides guidelines for patenting these elements, focusing on novelty, inventive step, and industrial applicability (EPO GL G-II, 5.2).
The patent landscape of mRNA therapeutics has proved a contentious and litigious one, as mRNA COVID-19 vaccine giants Moderna and Pfizer/BioNTech navigate infringement and invalidation proceedings around the world (Becker, 2024). Following the EPO’s interim decision (under appeal) revoking Moderna’s EP 3718565 patent in 2023, the EPO recently maintained another of the company’s key COVID-19 vaccine patents, EP 3590949, a decision which is also under appeal. Most recently, CureVac has achieved a victory at the EPO, where the Opposition Division upheld two important patents – EP 3708668 and EP4023755 – in amended form. Both decisions are still subject to appeal.
mRNA vaccines have also been considered by the EPO Boards of Appeal, including decision T 1639/21. This case concerned CureVac’s patent covering a combination of an mRNA vaccine and an anti-PD-1 antibody, with the central issue being whether the claimed synergistic effect rendered the invention non-obvious. The Board found that a synergistic effect of a combination does not automatically lead to inventive step, when the synergistic effect could have been reasonably expected in view of the prior art. The Board considered the mechanistic similarities between the combination disclosed in the patent and those of the prior art sufficient to invalidate the patent in question. If attempting to rely on a synergistic effect to show inventive step in order to obtain an mRNA therapeutic patent, it is therefore vital to also show that this synergistic effect is unexpected.
Summary
mRNA therapies represent a groundbreaking approach in modern medicine, offering unparalleled flexibility and potential across various therapeutic areas. The development of mRNA therapies involves intricate design, advanced delivery systems, and strategic modifications to enhance stability and efficacy. Patent protection is crucial for fostering innovation and ensuring the successful commercialisation of these therapies. As the field continues to evolve, mRNA therapies are poised to revolutionise the treatment landscape, providing new hope for patients with infectious diseases, cancer, and genetic disorders.
At GJE, our biotechnology group has extensive experience in patenting gene therapies and navigating the ever-increasing complexity of the accompanying IP landscape. To discuss your biotech IP strategy, please contact us at biotech@gje.com.
References
Asrorov, A. M., M. S. Ayubov, B. Tu, M. Shi, H. Wang et al. Coronavirus spike protein-based vaccines. Vaccine delivery systems. 2024. 24: 100198. Available online.
Baek, R., Coughlan, K., Jiang, L. et al. Characterizing the mechanism of action for mRNA therapeutics for the treatment of propionic acidemia, methylmalonic acidemia, and phenylketonuria. 2024. Nat Commun. 15: 3804. Available online.
Becker, Z. With EU agency’s ruling, Moderna tallies a win in vaccine patent war against Pfizer and BioNTech. 2024; accessed June 2025. Available online.
Hou, X., Zaks, T., Langer, R. et al. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021. 6, 1078–1094. Available online.
Moderna Clinical Trials. 2025; accessed June 2025. Available online.
Nobel Prize Press Release; accessed June 2025. Available online.
Qin, S., Tang, X., Chen, Y. et al. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Sig Transduct Target Ther. 2022.7, 166. Available online.
Weber J. S., et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): a randomised, phase 2b study. Lancet. 2024. 403(10427): 632-644. Available online.
EPO Guidelines for Examination, Part G, Chapter II, 5.2; accessed June 2025. Available online.
EP3718565 – Available online.
EP3590949 – Available online.
T 1639/21 – Available online.