Technology overview
Named after its ubiquitous presence in all eukaryotic cells, ubiquitin is a key regulator of many cellular processes. Its discovery in 1975 (Goldstein et al, 1975) marked the beginning of decades of research into protein degradation, signalling pathways, and cellular homeostasis, each of which can be modulated by the covalent attachment of ubiquitin onto a target protein via a process known as ubiquitination.
Ubiquitination is a post-translational modification which can tag proteins for degradation at the proteasome. The ubiquitination pathway firstly involves an enzyme known as E1, which ‘activates’ ubiquitin. A second enzyme, E2, receives the activated ubiquitin and awaits the final enzyme, the E3 ligase, which colocalises the target protein and the E2 enzyme such that ubiquitin can be conjugated onto a lysine residue of the target protein. The sequential addition of further ubiquitin molecules results in various polyubiquitin chain configurations, which act as the marker for degradation.
Growing insight into the regulation of proteins by ubiquitination has led to a spark in the development of targeted protein degradation therapies which exploit the sophisticated ubiquitination mechanism to selectively remove disease causing proteins.
Proteolysis targeting chimeras (PROTACs) are one example of targeted protein degradation therapies. PROTACs are a class of small-molecule drugs capable of simultaneously binding a target protein and an E3 ligase. By bringing the target protein in close proximity to the ligase, PROTACs induce ubiquitination of the target protein to mark it for degradation by the proteasome. Therefore, unlike classical inhibitors which block protein function, the heterobifunctionality of PROTACs allows for the selective degradation of target proteins, including those considered ‘undruggable’ or lesser-characterised.
Several disease-associated proteins may be considered undruggable, for instance due to drug resistant mutations, a lack of targetable catalytic activity (such as scaffold proteins), or intrinsic disorder (such as Tau). PROTACs circumvent these limitations by only requiring binding to a surface epitope with moderate affinity in order to bring the target protein into proximity with the E3 ligase. Additionally, following degradation of the target, the PROTAC remains available to continue targeting further proteins for destruction. Therefore, PROTACs could offer a safer alternative to classical inhibitors by demonstrating efficacy at lower doses.
Evidently, leveraging the ubiquitination pathway to target a diversity of disease-causing proteins for destruction has wide-reaching implications across a spectrum of indications. Since the generation of the first PROTAC molecule by Sakamoto and colleagues in 2001, selective degradation is increasingly attracting attention a promising therapeutic modality.
Indications treated
Although there are currently no PROTACs approved for clinical use, PROTACs represent a promising modality for targeted cancer therapy. Indeed, PROTACs have attracted substantial interest in the past decade. As of May 2025, 21 different PROTACs were advancing through clinical trials. In particular, Arvinas’ and Pfizer’s Vepdegestrant (ARV-471), an oestrogen receptor (E) degrader, was shown in Phase 3 trials to improve progression-free survival in oestrogen receptor-positive human epidermal growth factor receptor 2-negative (ER+/HER2-) breast cancer (NCT05654623). These breast cancer cells depend on oestrogen signalling via ER, and resistance mutations in the gene encoding ER are common. Therefore, PROTACs such as ARV-471 could offer more effective inhibition of ER-driven tumorigenesis, including in tumours which develop resistance.
Additionally, the androgen receptor (AR) is a central driver of prostate cancer biology, since prostate cells rely on androgens to grow and survive. Yet, clinical resistance to first-line androgen-deprivation therapies frequently occurs. Two further Arvinas PROTACs, Bavdegalutamide (ARV-110) (NCT03888612) and Luxdegalutamide (ARV-766) (NCT05067140) recently completed Phase 2 trials, demonstrating selective and potent AR degradation in metastatic-castration resistant prostate cancer.
There are also several ongoing Phase 2 trials for various indications including B-cell malignancies, solid tumours and skin abscesses.
Outside these clinical trials, PROTACs are being investigated for their applicability to a variety of different indications, including in frontotemporal dementia, where the heterobifunctionality of PROTACs could be exploited to induce degradation of tau aggregates (Silva et al, 2019). Studies have also discussed the promise of PROTACs in effectively managing viral infections by targeting proteins integral to viral replication (Mukerjee et al, 2024). PROTAC-adjacent technologies are also emerging, utilising for example glycan-mediated engagement of target proteins to achieve targeted destruction (Ganguly et al, 2024).
As such, this technology represents a powerful new class of therapeutics with broad potential across a multitude of diseases. The selective ability of PROTACs to destroy disease-causing proteins thus positions them as a highly promising next-generation modality.
Patent considerations
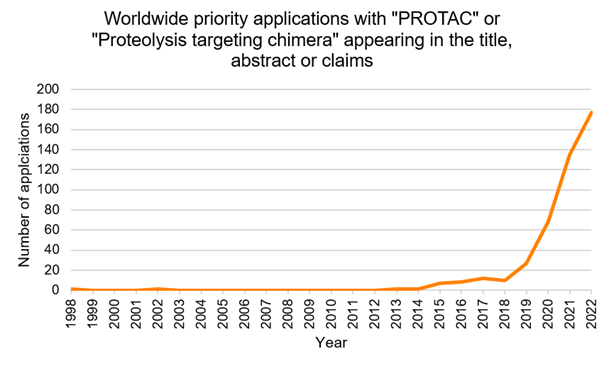
As a relatively new therapeutic modality, PROTAC-related patent filings in Europe have surged exponentially since 2019 (see the graph below), reflecting both their expansive therapeutic potential and growing commercial interest.
Unsurprisingly, the majority of granted claims in Europe are directed towards the chemical compounds themselves, alongside medical use claims. While broad claims are typical of early-stage patent landscapes, particularly in rapidly advancing fields like PROTACs, a few attempts have been made to functionally define the therapeutic use of these compounds. An example would be defining the pathology to be treated as any disease where degradation of the target is beneficial, or any disease mediated by the target. However, the European Patent Office is unlikely to grant such claims since it is challenging to determine exactly which diseases fall into the scope of such a claim (as seen in EP3286169 and EP3867263). Instead, outside of a first medical use claim (i.e., “a PROTAC for use in therapy”), it is advisable to direct subsequent medical use claims to particular diseases (i.e., “a PROTAC for use in the treatment of cancer”).
Care must also be taken to ensure that the two key binding moieties of the PROTAC (i.e., the part which binds to the E3 ligase and the part which binds to the target protein) are not disclosed in combination in the prior art, and that their combination is not obvious. It is possible that both binding elements are already known separately from previously developed ligands. Therefore, it the target-disease relationship is also known, this could raise questions as to the inventive step behind the claimed PROTAC – for instance, whether it is obvious to use the known target ligand in PROTAC format. It is therefore key to demonstrate a surprising or unexpected technical effect associated with the PROTAC, such as improved efficiency or selectivity.
Furthermore, where prodrugs and metabolites are claimed (or even featured in the description, as in the case of EP3867263), the application must disclose in sufficient detail which derivatives of the overarching compound possess suitable pharmacokinetic properties enabling them to release the active compound when administered in vivo.Providing a well-defined group of compounds together with compelling experimental evidence to support this group can therefore help avoid potential clarity and sufficiency issues.
Finally, as the PROTAC space continues to grow, claims will likely require narrowing. It is therefore advisable to include fall-back positions, such as claims directed to more specific chemical formulae, therapeutic indications to be treated, and in some cases dosage regimens, as fallback positions to help focus a broad claim to other commercially relevant embodiments.
Summary
PROTACs represent a highly promising therapeutic modality to a wide range of indications, especially where first-line treatments are becoming less effective. The commercial potential of a drug to treat otherwise ‘undruggable’ targets is significant. Therefore, as the field continues to expand, implementing an effective patent strategy to secure robust protection will be critical to supporting continued research, investment and development.
The biotechnology group at GJE has particular experience patenting a diverse range of biotechnological inventions in addition to advising innovative biotech companies and investors. . To discuss your biotech IP strategy, please contact us at biotech@gje.com.
References
Goldstein G, Scheid M, Hammerling U, Schlesinger DH, Niall HD, Boyse EA. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):11-5. Available online.
K.M. Sakamoto, K.B. Kim, A. Kumagai, F. Mercurio, C.M. Crews, & R.J. Deshaies, Protacs: Chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation, Proc. Natl. Acad. Sci. U.S.A. 98 (15) 8554-8559. Available online.
M Catarina Silva, Fleur M Ferguson, Quan Cai, Katherine A Donovan, Ghata Nandi, Debasis Patnaik, Tinghu Zhang, Hai-Tsang Huang, Diane E Lucente, Bradford C Dickerson, Timothy J Mitchison, Eric S Fischer, Nathanael S Gray, Stephen J Haggarty (2019) Targeted degradation of aberrant tau in frontotemporal dementia patient-derived neuronal cell models eLife 8:e45457. Available online.
Nobendu Mukerjee, Swastika Maitra, Arabinda Ghosh, Athanasios Alexiou, Nanasaheb D. Thorat, Exosome-mediated PROTAC delivery for treatment of RNA viral infections and zoonosis, Drug Discovery Today, 29(7):2024;104044. Available online.
Ganguly, Tanmoy; Back, Jonathan; Hanes, Melinda; Manni, Michela; Hesler, Stephen; Madala, Hanumantha Rao; Billion, Oaklyne; Oconnell, Courtney; Cotter, Kellie; Schelbert, Tina; Mally, Manuela; Sirena, Dominique Nicolas; Hillson, Jan Leslie; Kaundinya, Ganesh. Development of GE8820 for Selective and Rapid Removal of Pathogenic Autoantibodies in Primary Membranous Nephritis (PMN) and Other IgG4-Mediated Diseases: SA-PO733. Journal of the American Society of Nephrology 35(10S):2024. Available online.
EP3286169 – Available online.
EP3867263 – Available online.