Our top resource picks

Legal Update
Trade Mark Law & Practice at a Glance – February 2026
Read our February 2026 trade mark law and practice update, including updates from the UK, EU China, Canada and US
Learn more-
Legal Update
Trade Mark Law & Practice at a Glance – February 2026
Read our February 2026 trade mark law and practice update, including updates...
-
Legal Update
Patent Law & Practice at a Glance – February 2026
Read our February 2026 edition of patent law and practice which includes...
-
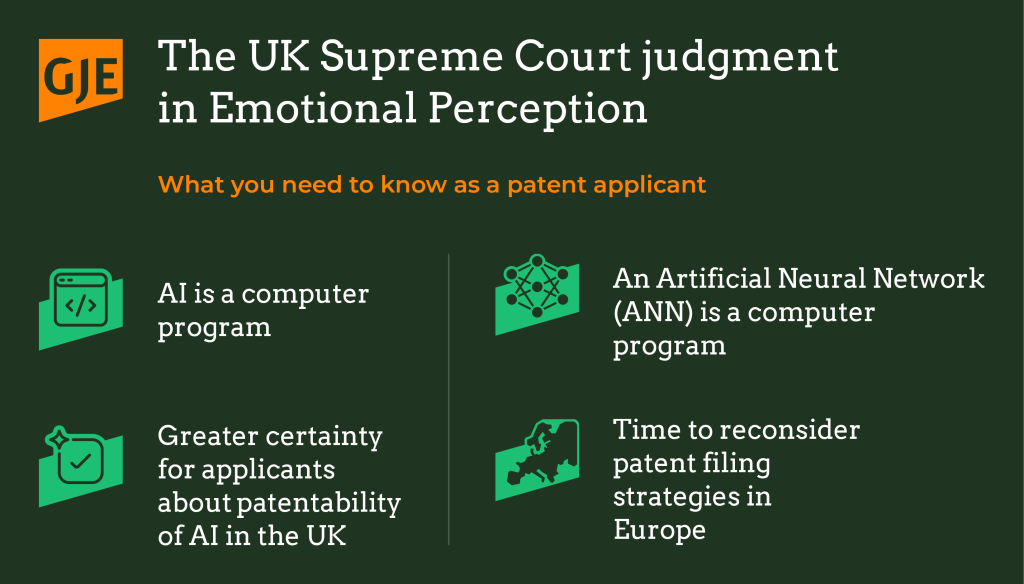
Article
An end to an emotional journey: The UK Supreme Court concludes the Emotional Perception saga
Today, the UK Supreme Court (UKSC) has delivered a historic, and much...
-
Article
EUIPO launches 2026 SME Fund
EU SMEs can access €7,000+ in IP funding through the EUIPO 2026...
All resources
Filters