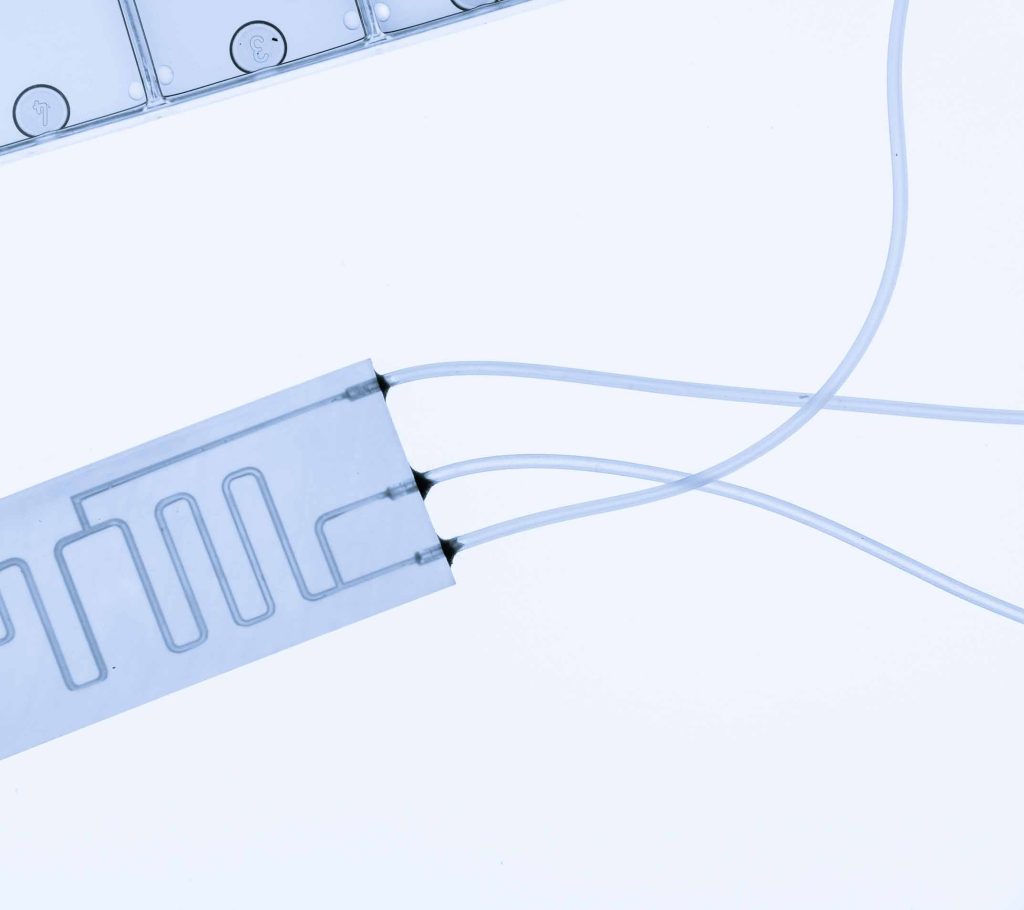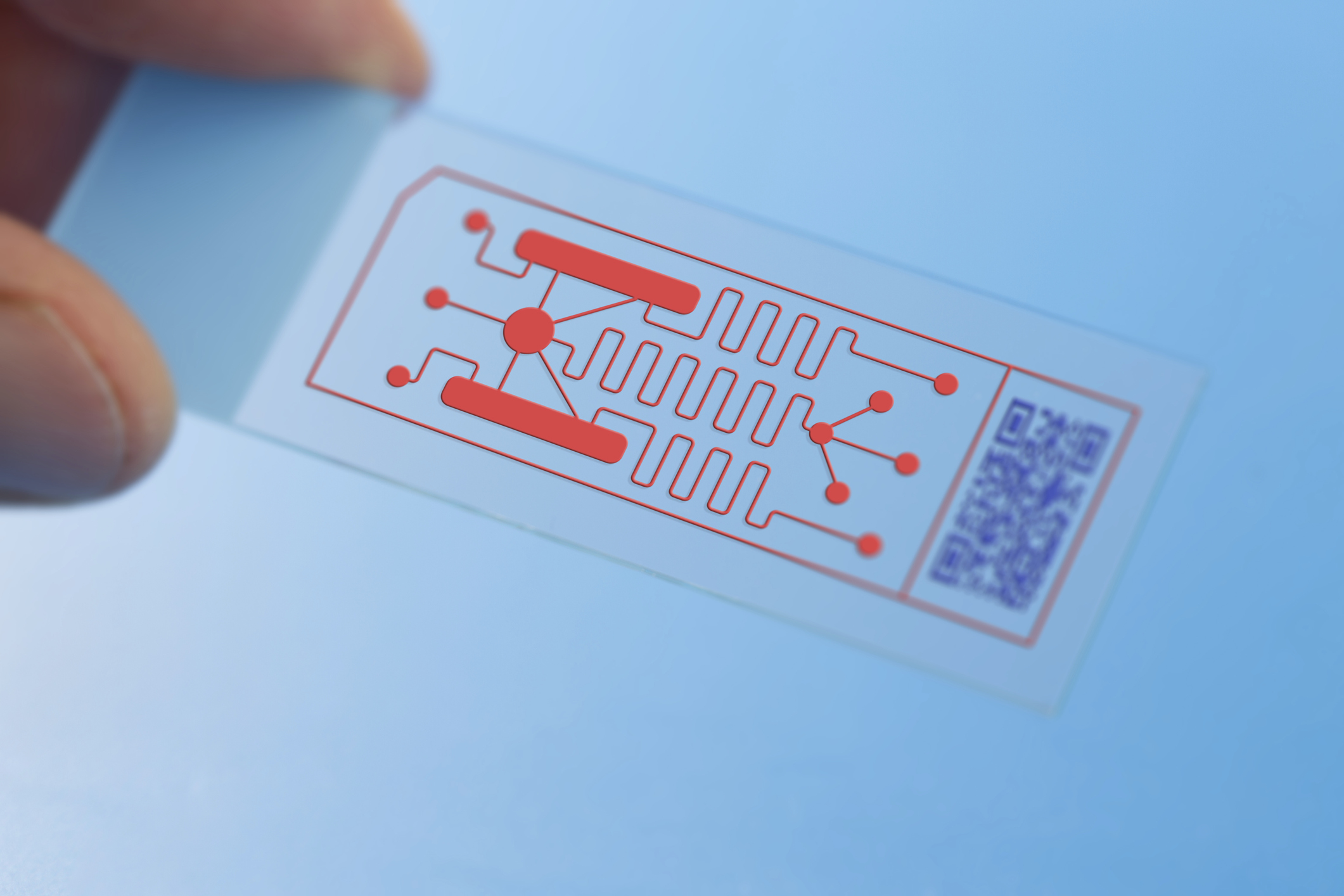
Organ on a Chip (OoaC) refers to the use of micro-devices that imitate human organs. It can allow for drugs to be tested in realistic environments, without the need for animal testing.
Our previous article reviews the technological and patent landscape of OoaC technology. Here we review future challenges from both a technical and patent perspective.
Technical challenges
It is well understood that modelling of biological tissue can be very challenging due to the inherent complexity and multifunctionality of human tissue. Different organs require a wide variety of manufacturing techniques in order to correctly simulate their individual behaviour. Due to the need for complex layered structures of membranes and fluidic channels, some OoaC devices utilise techniques such as 3D bioprinting to arrange biological material in space.
Cell sourcing presents other problems. Generally, OoaC tissues use cells derived from humans and animals or from commercially available cell lines, and in some cases use stem cells[a]. While each of these options provides certain advantages, no single cell source is perfect. Although commercially available cell lines allow for higher repeatability, they may not be as applicable to certain patients as primary cells from donors.
Even once a particular organ has been modelled, it is difficult to scale up manufacturing of the chips in a repeatable way. It is important that different drugs are tested fairly against each other, and it is often challenging to reliably manufacture large numbers of tissue chips to enable repeatable and consistent testing.
One further aim of OoaC is to emulate multi-organ systems, thereby creating what is known as a ‘body on a chip’. In principle, by modelling interactions between different organs, the overall system better reflects the target body, though such integration of organs is known to be challenging due to issues such as scaling and vascularisation[b].
Researchers involved with OoaC will frequently encounter technical challenges, such as those discussed above. Where solutions are found, patents are one way for the owners of the invention to benefit from the innovation. A patent may be granted for subject matter that is novel, inventive, and industrially applicable. Overcoming technical hurdles, such as those discussed above, is likely to involve novel and inventive approaches that satisfy these requirements. However, as will now be discussed, there are other patentability challenges that are relevant in research fields such as OoaC.
Patentability challenges
Those with experience in medical fields may be aware that various exclusions exist for patents concerning medical and biological inventions as well as for inventions that utilise stem cells. While these exclusions may initially sound problematic in a field such as OoaC, it is beneficial to study the scope of what is actually prohibited.
Stem cells
Under UK and European patent law[c], a patent application may be rejected for being contrary to public policy or morality. This extends to uses of human embryos for industrial or commercial purposes, such as the use of embryonic stem cells. This exclusion prevents patent protection for any product that may only be obtained by the destruction of human embryos. Therefore, the use of foetal and post-natal human cells may be allowable, and where embryonic stem cells are derived from parthenogenetically activated human oocytes, the exclusion does not apply[d].
In OoaC research, the stem cells are typically induced pluripotent stem cells (iPS), which are derived from adult tissues rather than from embryonic stem cells. Therefore, any innovations making use of this type of stem cell will not be prevented from obtaining patent protection.
Biological tissue
The core aim of OoaC technology is to produce realistic artificial organs. There exists a broad patentability exclusion under European patent law[e] for animal varieties and for essentially biological processes for the production of animals, which is largely in place to prevent patents being obtained for breeding techniques.
However, the Biotechnology Directive[f] clarifies that this exclusion shall be without prejudice to the patentability of inventions that concern a microbiological or another technical process or a product obtained by means of such a process, and that biological material isolated from its natural environment or produced using a technical process may be the subject of an invention, even if it previously occurred in nature. Therefore, realistic biological tissue produced using technical processes such as 3D bioprinting does not face an exclusion from patentability, even if the structure of the tissue is identical to the naturally occurring tissue[g].
Furthermore, whether claimed as a product or a product-by-process, the resulting tissue would still need to satisfy the requirements of novelty and inventive step in order for the claim to be allowed. Therefore, it is important that the resulting tissue is differentiated in a novel and non-obvious way from the target tissue. The way in which this is achieved may depend on the particulars of the manufacturing process, but it is conceivable that a technique such as 3D bioprinting may facilitate greater control over properties of the OoaC tissue.
Medical methods
Under UK and European patent law[h], a patent cannot be granted for a method of treatment of the human or animal body by surgery or therapy, or a method of diagnosis practised on the human or animal body. The reason for this exclusion is to ensure that those who carry out such methods as part of the medical treatment of humans or animals should not be inhibited by patents[i]. The exclusions act to remove any obstacles to the freedom to choose the best medical treatment to be applied to a patient and to avoid any delay in the application of such medical treatment[j].
Notably, these exclusions apply only to methods, so do not exclude any apparatus, device, substance, or composition that may be used in such a method. Given that OoaC technology is likely to result in the production of devices and products, this exclusion should not present any barrier towards obtaining patent protection.
Whether a particular method falls under the exclusion is decided on a case-by-case basis. A number of factors are taken into account, such as the need for professional skill, the degree of health risk, and the level of invasiveness of the procedure. For example, injections, tattoos, and piercings are not considered to be surgery, but the removal, treatment, and replacement of blood may be considered as a therapy or surgery[k].
It is important to note that these exclusions only apply if the method is practiced ‘on’ or ‘in’ the human or animal[l], and the method must be performed on a living human or animal. In other words, there must be some interaction with the body of the human or animal. Methods involving samples that remain external and removed from the body are not excluded.
Therefore, even when an OoaC tissue is derived from human or animal material, any method that occurs outside of the body will not be excluded from patentability. Furthermore, even if the OoaC tissue provides useful toxicology data for a particular treatment, the methods involving the tissue itself would not fall within the exclusion.
Concluding remarks
In view of the above, those working in the field of OoaC should not be deterred by these challenges and patentability exclusions. Businesses and researchers working in this field would, however, be well-advised to engage a patent attorney to ensure that all is being done to adequately protect their innovation, while simultaneously avoiding any potential intellectual property pitfalls.
If you would like to discuss your innovation further, please get in touch with our biomedical engineering team: biomedical.engineering@gje.com. The author’s details can be found here.
[a] Low, L.A., Mummery, C., Berridge, B.R. et al. Organs-on-chips: into the next decade. Nat Rev Drug Discov 20, 345-361 (2021)
[b] DoYeun Park et al. Integrating Organs-on-Chips: Multiplexing, Scaling, Vascularization, and Innervation. Trends in Biotechnology 38, 1 (2020)
[c] Section 1(3) of the Patents Act 1977, and Article 53(a) of the European Patent Convention
[d] T385/14
[e] Article 53(b) of the European Patent Convention
[f] Directive 98/44/EC
[g] Althabhawi N.M., Zainol Z.A. The Patent Eligibility of 3D Bioprinting: Towards a New Version of Living Inventions’ Patentability. Biomolecules 12, 124 (2022)
[h] Section 4A of the Patents Act 1977, and Article 53(c) of the European Patent Convention
[i] T116/85; G1/04
[j] G1/07
[k] T1075/06; T1695/07
[l] T58/87; T254/87